支鏈α-酮酸脫氫酶複合物
| A+醫學百科 >> 支鏈α-酮酸脫氫酶複合物 |
支鏈α-酮酸脫氫酶複合物(branched-chain α-ketoacid dehydrogenase complex,BCKDC)是一種在粒線體內膜中找到的酶之多次單元複合物(multi-subunit complex)。[1] 這種酶複合物催化具支鏈的短鏈α-酮酸的氧化脫羧反應。BCKDC是α-酮酸脫氫酶複合體家族內的成員,包括丙酮酸脫氫酶複合體(pyruvate dehydrogenase)和α-酮戊二酸脫氫酶複合體(α-ketoglutarate dehydrogenase),是三羧酸循環具有重要功能的酶。
目錄 |
輔助因子
這個複合物的需要下列5個輔因子(cofactor):
- 硫胺素焦磷酸(TPP)
- 黃素腺嘌呤二核苷酸(FAD)
- 煙醯胺腺嘌呤二核苷酸(NAD+)
- 硫辛酸(Lipoate)
- 輔酶A(Coenzyme A)
生物上的功能
在動物組織中,BCKDC催化不可逆步驟[2] ,分解代謝的支鏈胺基酸,即L-異白胺酸,L-纈氨酸和L-白胺酸及其衍生物(分別是L-α-酮-β-甲基戊酸酯,α-酮異戊酸和α-酮異己酸鹽)。[3][4][5] 在細菌中,這種酶參與的支鏈、長鏈脂肪酸的合成;[6]在植物中,這種酶是參與合成支鏈、長鏈的烴。
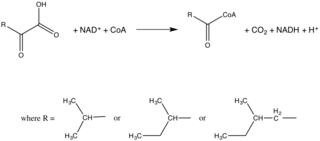
BCKDC催化的分解代謝之全反應示於圖1。
結構
BCKDC的酶催化機制很大程度上取決於這個大型酶複合體的精細結構。這種酶複合物是由三個催化次單元組成: α-酮酸脫氫酶(alpha-ketoacid dehydrogenase)(E1部分),二氫硫辛酸轉乙醯基酶(dihydrolipoyl transacylase) (E2部分),和二氫硫辛醯胺脫氫酶(dihydrolipoamide dehydrogenase)(E3部分)。在人體中的BCKDC核心中,有24個E2部分以八面體對稱排列。[7]24 E2次單元聚合物分兩部分,12個E1α2β2四聚體和6個E3同二聚體以非共價方式結合。除了E1/E3-結合區域,在E2次單元上,有2個其他重要的結構區域:
(i)以該蛋白質的氨基末端部分的硫辛醯-軸承結構域(lipoyl-bearing domain)
(ii)在蛋白質羧基端的核心區域(inner-core domain)。
核心區域是由兩個區間片段(連接子)連接到E2次單元的其他兩個區域。[9]核心區域對形成酶複合物的低聚核(oligomeric core)和催化醯基轉移酶的反應是必須的(由「機制」一節中所示)。[10] E2的硫辛醯區域可藉由上述提到連結子的彈性構像,使其在組裝好的BCKDC上之E1、E2和E3次單元的活性區位間自由擺動。(參照圖2)[11][12]因此就功能及結構方面來說,E2部分在BCKDC催化的整個反應扮演著重要的角色。
每個子單元的作用如下
E1次單元
E1採用硫胺素焦磷酸(TPP)作為催化的輔助因子。 E1催化α-酮酸的脫羧反應和後來的硫辛醯結構部分,以共價結合於E2次單元的還原反應(另一催化輔因子)。
E2次單元
E2催化醯基(acyl group)從硫辛醯部分轉至的輔酶A(化學計量的輔因子,stoichiometric cofactor)。[13]
E3次單元
在E33部分是黃素蛋白(flavoprotein),且其可作為氧化劑並利用FAD(催化輔因子)重新氧化還原E2次單元上的硫辛醯硫部分(lipoyl sulfur residues);然後FAD將這些質子和電子轉移到NAD+(化學計量的輔因子,stoichiometric cofactor)以完成反應循環。
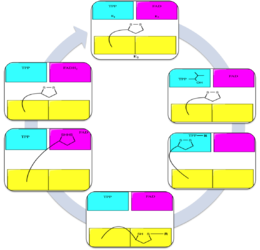
機制
如前面提到的,在哺乳類動物體內的BCKDC主要功能是,催化支鏈胺基酸分解代謝反應中的不可逆步驟。然而,BCKDC具有相對廣泛的特異性,在比較比例(comparable rates)及對支鏈胺基酸的基質之Km值相似情況下,也可氧化4-甲硫基-2-氧代丁酸(4-methylthio-2-oxobutyrate)和2-氧代丁酸(2-oxobutyrate)。[14]BCKDC也可氧化丙酮酸(pyruvate),但這種緩慢速度下,副反應只小具生理意義。[15][16]
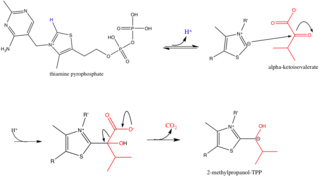
其反應機理如下所示。[17] 請注意,任何一種支鏈 α-酮酸可以作為起始原料;在這個例子中,α-酮異戊酸任意地被選作為BCKDC的基質。
- 注意:步驟1和2在E1區域發生。
步驟1: α-酮異戊酸結合TPP,然後進行脫羧反應(decarboxylated),適當的箭頭推動機構示於圖3。
步驟2:將2-甲基丙醇-TPP(2-methylpropanol-TPP)被氧化形成乙醯基(acetyl group)而被同時轉移到E2中的硫辛醯輔酶。注意,TPP被再生。適當的箭頭推動機構示於圖4。
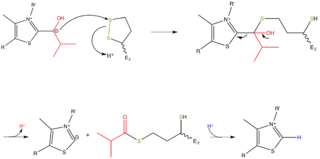
- 註:醯化硫辛醯臂(acylated lipoyl arm)現在離開E1,並盪到E2的活性區位,在此處發生第3步驟。
步驟3:醯基(Acyl group)轉移到輔酶A(CoA)。適當的箭頭推動機構示於圖5。
- 註:被還原硫辛醯臂現在盪到在E3的活性區位,此處步驟4和5發生。
步驟4:將硫辛醯區域被FAD輔酶氧化,如圖所示於圖6。
步驟5: FADH2再氧化成FAD,產生NADH:NADH::FADH2 + NAD+ --> FAD + NADH + H+
疾病相關
缺乏任何這種復酶複合物以或複合物的抑制,會使支鏈胺基酸和它們有害衍生物在體內積聚。這些積累會產生有甜味的排泄物(如耳垢和尿液),及病理學常稱為楓糖尿症。[18]
在原發性膽汁性肝硬化中,[一種急性肝功能衰竭(acute liver failure)],這種酶是自身抗原(autoantigen),這些抗體(antibodies)會辨識氧化的蛋白質,導致炎症免疫反應,有些發炎反應可由麩質過敏解釋。[19] 其他粒線體自身抗原,可由抗粒線體抗體(anti-mitochondrial antibodies)所辨識的抗原,包括丙酮酸脫氫酶(pyruvate dehydrogenase)和支鏈酮戊二酸脫氫酶(oxoglutarate dehydrogenase)。
參考
- ↑ Indo I, Kitano A, Endo F, Akaboshi I, Matsuda, I. Altered Kinetic Properties of the Branched-Chain Alpha-Keto Acid Dehydrogenase Complex Due to Mutation of the Beta-Subunit of the Branched-Chain Alpha-Keto Acid Decarboxylase (E1) Component in Lymphoblastoid Cells Derived from Patients with Maple Syrup Urine Disease. J Clin Invest. 1987, 80 (1): 63–70. doi:10.1172/JCI113064. PMID 3597778.
- ↑ Yeaman SJ. The 2-oxo acid dehydrogenase complexes: recent advances.. Biochem J.. 1989, 257 (3): 625–632. PMID 2649080.
- ↑ Broquist HP, Trupin JS.. Amino Acid Metabolism. Annual Review of Biochemistry. 1966, 35: 231–247. doi:10.1146/annurev.bi.35.070166.001311.
- ↑ Harris RA, Paxton R, Powell SM, Goodwin GW, Kuntz MJ, Han AC.. Regulation of branched-chain alpha-ketoacid dehydrogenase complex by covalent modification.. Adv Enzyme Regul.. 1986, 25: 219–237. doi:10.1016/0065-2571(86)90016-6. PMID 3028049.
- ↑ Namba Y, Yoshizawa K, Ejima A, Hayashi T, Kaneda T.. Coenzyme A- and nicotinamide adenine dinucleotide-dependent branched chain alpha-keto acid dehydrogenase. I. Purification and properties of the enzyme from Bacillus subtilis.. J Biol Chem.. 1969, 244 (16): 4437–4447. PMID 4308861.
- ↑ Lennarz WJ, et al.. The role of isoleucine in the biosynthesis of branched-chain fatty acids by micrococcus lysodeikticus.. Biochemical and Biophysical Research Communications. 1961, 6 (2): 1112–116. doi:10.1016/0006-291X(61)90395-3. PMID 14463994.
- ↑ Aevarsson A, Chuang JL, Wynn RM, Turley S, Chuang DT, Hol WGJ.. Crystal structure of human branched-chain α-ketoacid dehydrogenase and the molecular basis of multienzyme complex deficiency in maple syrup urine disease. Structure. 2000, 8 (3): 277–291. doi:10.1016/S0969-2126(00)00105-2. PMID 10745006.
- ↑ Berg, Jeremy M., John L. Tymoczko, Lubert Stryer, and Lubert Stryer. Biochemistry. 6th ed. New York: W.H. Freeman, 2007. 481. Print.
- ↑ Chuang DT.. Molecular studies of mammalian branched-chain alpha-keto acid dehydrogenase complexes: domain structures, expression, and inborn errors.. Annals of the New York Academy of Sciences. 1989, 573: 137–154. doi:10.1111/j.1749-6632.1989.tb14992.x. PMID 2699394.
- ↑ Chuang DT, Hu CW, Ku LS, Markovitz PJ, Cox RP.. Subunit structure of the dihydrolipoyl transacylase component of branched-chain alpha-keto acid dehydrogenase complex from bovine liver. Characterization of the inner transacylase core.. J Biol Chem. 1985, 260 (25): 13779–86. PMID 4055756.
- ↑ Reed LJ, Hackert ML.. Structure-function relationships in dihydrolipoamide acyltransferases.. J Biol Chem. 1990, 265 (16): 8971–8974. PMID 2188967.
- ↑ Perham RN.. Domains, motifs, and linkers in 2-oxo acid dehydrogenase multienzyme complexes: a paradigm in the design of a multifunctional protein.. Biochemistry. 1991, 30 (35): 8501–8512. doi:10.1021/bi00099a001. PMID 1888719.
- ↑ Heffelfinger SC, Sewell ET, Danner DJ.. Identification of specific subunits of highly purified bovine liver branched-chain ketoacid dehydrogenase.. Biochemistry. 1983, 22 (24): 5519–5522. doi:10.1021/bi00293a011. PMID 6652074.
- ↑ Jones SM, Yeaman SJ.. [http//www.ncbi.nlm.nih.gov/pmc/articles/PMC1147032/ Oxidative decarboxylation of 4-methylthio-2-oxobutyrate by branched-chain 2-oxo acid dehydrogenase complex.]. Biochemistry. 1986, 237 (2): 621–623. PMID 3800905. PMC 1147032.
- ↑ Pettit FH, Yeaman SJ, Reed LJ.. [http//www.ncbi.nlm.nih.gov/pmc/articles/PMC336225/ Purification and characterization of branched chain alpha-keto acid dehydrogenase complex of bovine kidney.]. Proceedings of the National Academy of Sciences of the United States of America. 1978, 75 (10): 4881–4885. doi:10.1073/pnas.75.10.4881. PMID 283398. PMC 336225.
- ↑ Damuni Z, Merryfield ML, Humphreys JS, Reed LJ.. [http//www.ncbi.nlm.nih.gov/pmc/articles/PMC345583/ Purification and properties of branched-chain alpha-keto acid dehydrogenase phosphatase from bovine kidney.]. Proceedings of the National Academy of Sciences of the United States of America. 1984, 81 (14): 4335–4338. doi:10.1073/pnas.81.14.4335. PMID 6589597. PMC 345583.
- ↑ Berg, Jeremy M., John L. Tymoczko, Lubert Stryer, and Lubert Stryer. Biochemistry. 6th ed. New York: W.H. Freeman, 2007. 478-79. Print.
- ↑ Podebrad F, Heil M, Reichert S, Mosandl A, Sewell AC, Böhles H. 4,5-dimethyl-3-hydroxy-25H-furanone (sotolone)--the odour of maple syrup urine disease. Journal of Inherited Metabolic Disease. April 1999, 22 (2): 107–114. doi:10.1023/A:1005433516026. PMID 10234605.
- ↑ Leung PS, Rossaro L, Davis PA, et al.. Antimitochondrial antibodies in acute liver failure: Implications for primary biliary cirrhosis. Hepatology. 2007, 46 (5): 1436–42. doi:10.1002/hep.21828. PMID 17657817.
外部連結
- GeneReviews/NCBI/NIH/UW entry on Maple Syrup Urine Disease
- MeSH(醫學主題詞)上面的Branched+Chain+Ketoacid+Dehydrogenase
- EC 1.2.4.4
- [1]
|
|||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||
|
|||||||||||||||||||||||
參考來源
| 關於「支鏈α-酮酸脫氫酶複合物」的留言: | |
|
目前暫無留言 | |
| 添加留言 | |