諾維喬克
| A+醫學百科 >> 諾維喬克 |
諾維喬克(俄文новичок,Novichok)是蘇聯在1970年代及1980年代開發的一系列神經毒劑[1]。據稱有些衍生物的毒性可能是 VX (神經毒劑)的五至八倍,但無法得到證實[2][3]。諾維喬克屬於蘇聯「Foliant」計劃中開發的第四代化學武器[4],最早的名稱是K-84,後來更名為A-230。諾維喬克系列包括超過一百種不同結構的變體[5],其中最有軍事價值[來源請求]的是A-232(Novichok-5;[(2-chloro-1-methylethoxy)fluorohydroxy phosphinyl]oxy]carbonimidic chloride fluoride];氟磷酸氟氯二取代亞甲胺基(2-氯)異丙基酯),其次是A-230(Novichok-7;(2-chloroethoxy)fluorohydroxyphosphinyl]oxy] carbonimidic chloride fluoride;氟磷酸氟氯二取代亞甲胺基(2-氯)乙基酯)。
在1980年蘇聯化學家米爾査揚諾夫在《國家的秘密:俄羅斯化學武器計劃內部知情人士的記述》一書中公開諾維喬克結構。[來源請求]Steven L. Hoenig在《化學與生物戰劑手冊》第二版中亦列出了部分Novichok家族成員的分子結構。
化學結構
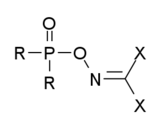
有二種有機磷製劑都被歸類為諾維喬克毒劑。第一種是有dihaloformaldoxime基團的有機磷化合物,其通式如下:其中的R 可以是烷基、烷氧基、烷氨基或氟,而X為鹵素(氟、氯、溴)或是像C≡N等擬鹵素。這些化合物被廣泛地記載在當時的蘇聯文獻中,但不確定是否包括所有的諾維喬克毒劑[6][7][8][9][10][11][12][13]。
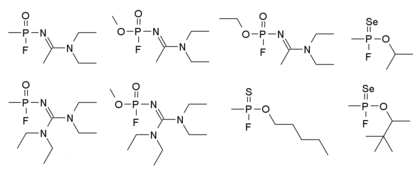
Mirzayanov在自傳中有提到另一種結構的諾維喬克諾維喬克毒劑,其結構如下:他明確的製作了大量的化合物,許多毒性較低的衍生物也在公開文獻中以新型有機磷殺蟲劑的方式提及,因此秘密的化學武器研究可以偽裝為合法的農藥研究[14]。
參考資料
- ↑ Tucker, J. B.; War of Nerves; Anchor Books; New York; 2006; pp 232-233.
- ↑ Vadim J. Birstein. The Perversion Of Knowledge: The True Story of Soviet Science. Westview Press (2004) ISBN 0-8133-4280-5
- ↑ Yevgenia Albats and Catherine A. Fitzpatrick. The State Within a State: The KGB and Its Hold on Russia — Past, Present, and Future, 1994. ISBN 0-374-18104-7 (see pages 325-328)
- ↑ Tucker, J. B.; War of Nerves; Anchor Books; New York; 2006; pp 231.
- ↑ Tucker, J. B.; War of Nerves; Anchor Books; New York; 2006; pp 233.
- ↑ Kruglyak Yu L, Malekin SI, Martynov IV. Phosphorylated oximes. XII. Reactions of 2-halophospholanes with dichlorofluoronitrosomethane. Zhurnal Obshchei Khimii. 1972; 42(4):811-14.
- ↑ Raevskii OA, Chapysheva NV, Ivanov AN, Sokolov VB, Martynov IV. Effect of Alkyl Substituents in Phosphorylated Oximes. Zhurnal Obshchei Khimii. 1987; 57(12):2720-2723
- ↑ Raevskii OA, Grigor'ev V Yu, Solov'ev VP, Ivanov AN, Sokolov VB, Martynov IV. Electron-Donor Functions of Ethyl Methylchloroformimino Methylphosphonate. Zhurnal Obshchei Khimii. 1987; 57(9):2073-2078
- ↑ Makhaeva GF, Filonenko IV, Yankovskaya VL, Fomicheva SB, Malygin VV. Comparative studies of O,O-dialkyl-O-chloromethylchloroformimino phosphates: interaction with neuropathy target esterase and acetylcholinesterase. Neurotoxicology. 1998 Aug-Oct;19(4-5):623-8. PMID 9745921
- ↑ Raevskiĭ OA, Chistiakov VV, Agabekian RS, Sapegin AM, Zefirov NS. Formation of models of the interaction between organophosphate compound structure and their ability to inhibit cholinesterase. Bioorganicheskaia Khimiia. 1990 Nov;16(11):1509-22. PMID 2096825
- ↑ Ivanov IuIa, Sokolov VB, Epishina TA, Martynov IV. O-substituted alkylchloroformoximes as substrates and inhibitors of cholinesterases. Doklady Akademii Nauk SSSR. 1990;310(5):1253-5. PMID 2354654
- ↑ Malygin VV, Sokolov VB, Richardson RJ, Makhaeva GF. Quantitative structure-activity relationships predict the delayed neurotoxicity potential of a series of O-alkyl-O-methylchloroformimino phenylphosphonates. Journal of Toxicology and Environmental Health Part A. 2003 Apr 11;66(7):611-25. PMID 12746136
- ↑ Steven L. Hoenig. Compendium of Chemical Warfare Agents. Springer New York, 2007. ISBN 978-0-387-34626-7
- ↑ Vil S Mirzayanov. State Secrets. An Insider's Chronicle of the Russian Chemical Weapons Program. (2009) pp142-145, 179-180. ISBN 978-1-4327-2566-2
|
|||||||||||||||||||||||||||||||||||||||||||||||
參考來源
| 關於「諾維喬克」的留言: | |
|
目前暫無留言 | |
| 添加留言 | |