Riboflavin kinase
核黃素激酶 |
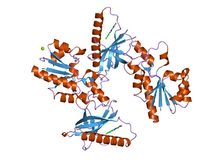 |
| crystal structure of flavin binding to fad synthetase from thermotoga maritina |
| 鑒定 |
| 標誌 |
Flavokinase |
| Pfam(蛋白家族查詢站) |
PF01687 |
| InterPro(蛋白數據整合站) |
IPR015865 |
| SCOP(蛋白結構分類數據站) |
1mrz |
|
|
核黃素激酶(英語:riboflavin kinase,EC 2.7.1.26)是一個催化以下化學反應的酶:
- ATP + 核黃素
 ADP + FMN
ADP + FMN
該酶催化的反應的底物為ATP和核黃素,產物是ADP和黃素單核苷酸(FMN)。
但是,在古菌核黃素激酶(EC 2.7.1.161)中,常使用CTP而非ATP作為反應底物,催化如下反應:
- CTP + 核黃素
 CDP + FMN [2]
CDP + FMN [2]
核黃素激酶也在許多細菌中發現,具有類似的功能,但存在若干數量的胺基酸不同。
反應
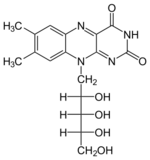 + XTP →
+ XTP → 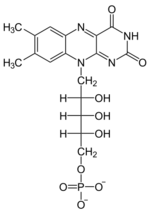 + XDP
+ XDP
Riboflavin is converted into catalytically active cofactors (FAD and FMN) by the actions of riboflavin kinase (EC 2.7.1.26), which converts it into FMN, and FAD synthetase (EC 2.7.7.2), which adenylates FMN to FAD. Eukaryotes usually have two separate enzymes, while most prokaryotes have a single bifunctional protein that can carry out both catalyses, although exceptions occur in both cases. While eukaryotic monofunctional riboflavin kinase is orthologous to the bifunctional prokaryotic enzyme,[3] the monofunctional FAD synthetase differs from its prokaryotic counterpart, and is instead related to the PAPS-reductase family.[4] The bacterial FAD synthetase that is part of the bifunctional enzyme has remote similarity to nucleotidyl transferases and, hence, it may be involved in the adenylylation reaction of FAD synthetases.[5]
This enzyme belongs to the family of transferases, to be specific, those transferring phosphorus-containing groups (phosphotransferases) with an alcohol group as acceptor. The systematic name of this enzyme class is ATP:riboflavin 5'-phosphotransferase. This enzyme is also called flavokinase. This enzyme participates in riboflavin metabolism.
結構
截止2007年底,這類酶中已有14個三級結構被解決,在PDB的登陸代碼為1N05,1N06,1N07,1N08,1NB0,1NB9,1P4M,1Q9S,2P3M,2VBS,2VBT,3CTA,2VBU,2VBV。
參考文獻
- ↑ PDB 3CTA; Bonanno, J.B., Rutter, M., Bain, K.T., Mendoza, M., Romero, R., Smith, D., Wasserman, S., Sauder, J.M., Burley, S.K., Almo, S.C.. Crystal structure of riboflavin kinase from Thermoplasma acidophilum. 2008.
- ↑ Ammelburg M, Hartmann MD, Djuranovic S, Alva V, Koretke KK, Martin J, Sauer G, Truffault V, Zeth K, Lupas AN, Coles M. A CTP-Dependent Archaeal Riboflavin Kinase Forms a Bridge in the Evolution of Cradle-Loop Barrels. Structure.. 2007, 12 (12): 1577–90. doi:10.1016/j.str.2007.09.027. PMID 18073108.
- ↑ Osterman AL, Zhang H, Zhou Q, Karthikeyan S. Ligand binding-induced conformational changes in riboflavin kinase: structural basis for the ordered mechanism. Biochemistry. 2003, 42 (43): 12532–8. doi:10.1021/bi035450t. PMID 14580199.
- ↑ Galluccio M, Brizio C, Torchetti EM, Ferranti P, Gianazza E, Indiveri C, Barile M. Over-expression in Escherichia coli, purification and characterization of isoform 2 of human FAD synthetase. Protein Expr. Purif.. 2007, 52 (1): 175–81. doi:10.1016/j.pep.2006.09.002. PMID 17049878.
- ↑ Srinivasan N, Krupa A, Sandhya K, Jonnalagadda S. A conserved domain in prokaryotic bifunctional FAD synthetases can potentially catalyze nucleotide transfer. Trends Biochem. Sci.. 2003, 28 (1): 9–12. doi:10.1016/S0968-0004(02)00009-9. PMID 12517446.
延伸閱讀
- CHASSY BM, ARSENIS C, MCCORMICK DB. THE EFFECT OF THE LENGTH OF THE SIDE CHAIN OF FLAVINS ON REACTIVITY WITH FLAVOKINASE. J. Biol. Chem.. 1965, 240: 1338–40. PMID 14284745.
- GIRI KV, KRISHNASWAMY PR, RAO NA. [http//www.ncbi.nlm.nih.gov/pmc/articles/PMC1196627/ Studies on plant flavokinase]. Biochem. J.. 1958, 70 (1): 66–71. PMID 13584303. PMC 1196627.
- KEARNEY EB. The interaction of yeast flavokinase with riboflavin analogues. J. Biol. Chem.. 1952, 194 (2): 747–54. PMID 14927668.
- McCormick DB and Butler RC. Substrate specificity of liver flavokinase. Biochim. Biophys. Acta. 1962, 65 (2): 326–332. doi:10.1016/0006-3002(62)91051-X.
- Sandoval FJ, Roje S. An FMN hydrolase is fused to a riboflavin kinase homolog in plants. J. Biol. Chem.. 2005, 280 (46): 38337–45. doi:10.1074/jbc.M500350200. PMID 16183635.
- Solovieva IM, Tarasov KV, Perumov DA. Main physicochemical features of monofunctional flavokinase from Bacillus subtilis. B. Mosc, Biochemistry. (2): 177–81. PMID 12693963.
- Solovieva IM, Kreneva RA, Leak DJ, Perumov DA. The ribR gene encodes a monofunctional riboflavin kinase which is involved in regulation of the Bacillus subtilis riboflavin operon. Microbiology.. Pt 1, 145: 67–73. doi:10.1099/13500872-145-1-67. PMID 10206712.
此條目包含有源於Pfam以及InterPro的屬於公有領域的文本 IPR015865
參考來源
更多醫學百科條目

 ADP + FMN
ADP + FMN CDP + FMN [2]
CDP + FMN [2]
